Sunday, March 16, 2008
Assignment 2 - Article Review
by Eran Rosines, Heidi J. Schmidt and Sanjay K. Nigam. Biomaterials 28(2007): 4806-4817.
The research presented in this article is very insightful and will lead to many future applications in medicinal research involving the kidneys. Studies prior to this one have shown that hyaluronic acid (HA), a glycosaminoglycan, plays a vital role in mammalian development. It has also been shown to be important for tissue engineering purposes as it is antigenic and immunocompatible. Use as an injectible gel for viscosupplementation of osteoarthritic joints and as a dermatological treatment for wrinkles represent only a couple of its many potential uses. The purpose of the article was to investigate the importance of HA during renal organogenesis. Many branching morphogenesis and mesenchymal-to-epithelial transformation (MET) regulatory mechanisms have already been identified for renal organogenesis, however, it is poorly understood how branching morphogenesis is stopped and collecting duct and nephron differentiation is started. It is, however, known that in the absence of HA murine embryos exhibit growth retardation and death within ten days on embryonic development suggesting an essential role for HA. The authors hope that the results presented in this paper will shed some light on this matter.
HA is synthesized by three HA synthase genes, has-1, has-2 and has-3 with has-2 accounting for at least 97% of HA synthesized during murine embryonic development. All genes synthesize HA in different molecular weights (MW). HA is known to exist at different MWs and concentrations during kidney differentiation. The MW of HA is controlled in vivo by degradative hyaluronidases including that encoded by hyal-2.
In order to demonstrate the potential importance of HA several kidney culture methods were utilized. These include qPCR to detect the expression of has-2 and hyal-2 during in vivo kidney development; several tests adding hyaluronidase and HA at various MWs and concentrations to in vitro metanephric and isolated uretic bud (UB) culture systems; morphometric analysis to quantify the effects on branching morphogenesis and; quantitative PCR of functional renal differentiation markers to measure UB/metanephric mesenchym (MM) differentiation.
The results of this paper show that HA has the ability to simultaneously modulate UB branching, promote MET, and promote differentiation of both MM and the UB depending on the concentration and MW of HA. The results also suggest that endogenous HA is required for branching morphogenesis as the presence of hyaluronidase inhibited branching morphogenesis in both UB and whole kidney cultures. The in vitro tests involving the use of various concentrations and MWs of HA revealed that HA stimulates branching morphogenesis at low concentrations and MW but inhibits branching at high concentrations and high MW. qPCR results showed that has-2 and hyal-2, which regulate the size of HA molecules, are highly expressed during kidney development. This, in combination with the other results presented in this paper, suggests that specific sizes and concentrations of HA may act to independently regulate UB branching and promote tubular maturation and thus may be responsible for ending branching morphogenesis and initiating nephron differentiation. Quantitative PCR of functional renal differentiation markers to measure UB/MM differentiation demonstrated that HA of a variety of MWs strongly promotes mesenchymal epithelialization and nephron differentiation in a concentration-dependent manner.
So what does all this mean??
These results suggest that HA may act as a growth factor sink for the UB but the viscous high concentration and high MW may in turn act as a physical barrier to the growth required for UB branching morphogenesis which could have tissue engineering and developmental relevance. Furthermore, it appears that HA acts as a switch molecule controlling when branching morphogenesis is stopped and when collecting duct and nephron differentiation is started; something little has been known about prior to this research. But, in my opinion, the greatest contribution of this research is the insight into the potential use of HA in renal repair, including promoting the formation new nephrons in adult kidneys with renal disease and the use of HA receptors or HA analogs as a new set of drug targets or therapies to promote renal tubule regeneration. HA may also promote tubule regeneration in injured or cryopreserved kidneys, which is very useful for kidney transplantation and can be used for creating a 3D scaffold for in vitro kidney engineering from developmental tissues.
The results presented in this paper appear to greatly support the authors claims of future applications of HA in kidney engineering. All of the figures were quite informative as they were very visual using color images to show the effects of HA and hyal-2 on branching. The data from the color images was also displayed in bar graphs providing two ways to visualize the results. All graphs and figures were well presented and easy to understand with informative figure legends. Overall, I found this paper moderately easy to understand. I really liked how the results section was split up to distinguish between all the tests. The discussion was kind of wordy at times and I did have to go back over some parts to fully understand but the gist of the research presented came through clearly.
Monday, March 3, 2008
Assignment 1 - The Kidneys: Description, Function and Pathology
 The kidneys, part of the urinary system, remove toxic by-products of metabolism from the bloodstream and urine from the body. They are reddish brown, bean-shaped organs located high in the abdominal cavity behind the peritoneum (the membrane that lines the abdomen). Concave on one long side and convex on the opposite, the upper end of each kidney is tilted slightly inward toward the backbone [1] Each kidney is about 11 cm long, 4 to 5 cm wide, and 2 to 3 cm thick. Originating from the intermediate mesoderm, the kidney, which is embedded in perineal fat, lies with its convex border situated laterally and its concave hilum, a deep vertical cleft, facing medially. This is the point of entry and exit of the renal arteries and veins, lymphatic vessels, nerves, and the enlarged upper end of the ureters [2]. The renal pelvis, expanded upper end of the ureter, is divided into 2 or 3 major calyces which in turn are further divided into several small branches, the minor calyces. An extension of the hilum deeper into the kidney is called the renal sinus [3]. The sinus itself is largely filled with loose connective tissue and adipose tissue [4].
The kidneys, part of the urinary system, remove toxic by-products of metabolism from the bloodstream and urine from the body. They are reddish brown, bean-shaped organs located high in the abdominal cavity behind the peritoneum (the membrane that lines the abdomen). Concave on one long side and convex on the opposite, the upper end of each kidney is tilted slightly inward toward the backbone [1] Each kidney is about 11 cm long, 4 to 5 cm wide, and 2 to 3 cm thick. Originating from the intermediate mesoderm, the kidney, which is embedded in perineal fat, lies with its convex border situated laterally and its concave hilum, a deep vertical cleft, facing medially. This is the point of entry and exit of the renal arteries and veins, lymphatic vessels, nerves, and the enlarged upper end of the ureters [2]. The renal pelvis, expanded upper end of the ureter, is divided into 2 or 3 major calyces which in turn are further divided into several small branches, the minor calyces. An extension of the hilum deeper into the kidney is called the renal sinus [3]. The sinus itself is largely filled with loose connective tissue and adipose tissue [4]. Overview of Kidney Structure
 Each kidney is covered by a thin fibrous capsule consisting mainly of dense, irregular collagenous connective tissue with occasional elastic fibers and smooth muscle cells [2]. The capsule, which passes inward at the hilum, forms the connective tissue covering of the sinus and becomes continuous with the connective tissue forming the walls of the renal pelvis and minor calyces. A hemisected view of the kidney reveals a darker, outer region, the cortex, and a paler, inner region, the medulla. The difference in color reflects the distribution of blood. About 90-95% of the blood passing through the kidney is in the cortex, the remaining 5-10% is in the medulla [4].
Each kidney is covered by a thin fibrous capsule consisting mainly of dense, irregular collagenous connective tissue with occasional elastic fibers and smooth muscle cells [2]. The capsule, which passes inward at the hilum, forms the connective tissue covering of the sinus and becomes continuous with the connective tissue forming the walls of the renal pelvis and minor calyces. A hemisected view of the kidney reveals a darker, outer region, the cortex, and a paler, inner region, the medulla. The difference in color reflects the distribution of blood. About 90-95% of the blood passing through the kidney is in the cortex, the remaining 5-10% is in the medulla [4].In humans, the renal medulla consists of 10-18 conical or pyramidal structures, the medullary pyramids (also referred to as renal pyramids)which contain the nephric tubules. The apical portion - called the papilla- of each medullary pyramid projects into a minor calyx. The tip of the papilla is perforated with the openings of 10-25 papillary ducts, the final collecting ducts of the uriniferous tubules, and is therefore called the area cribrosa (Latin for sieve-like) [4].Parallel arrays of tubules, the medullary rays, arise from the base of the medullary pyramids and penetrate the cortex. Neighboring pyramids are separated from each other by material resembling the cortex, the cortical columns. The portion of the cortex overlying the base of each pyramid is known as a cortical arch. A renal pyramid, with its associated cortical arch and cortical columns, represents a lobe of the kidney [2].
The connective tissue of the kidney parenchyma is called interstitial tissue. It increases in amount from the cortex, where it constitutes about 7% of the volume, to the inner region of the medulla, where it constitutes about 20% of the volume [4]. Two kinds of interstitial cells are found in the cortex. The first kind, found between the basement membrane of the tubules and the adjacent peritubular capillaries (to be discussed), resembles fibroblasts and secretes the collagen and glycosaminoglycans of the extracellular matrix of the interstitium. The second type of cell is a macrophage [5].
The principal interstitial cells in the medulla resemble myofibroblasts. They contain prominent bundles of actin filaments, abundant rough ER, well-developed Golgi, lysosomes and lipid droplets [4]. It is thought these cells are the precursors of prostaglandins [5].
The Uriniferous Tubules - The Functional Unit of the Kidney
 The functional unit of the kidney is the uriniferous tubule, a highly convoluted structure that modifies the fluid passing through it to form to form urine as its final output. This tubule consists of two parts, the nephron (Gr. nephros kidney) and the collecting tubule. There are approximately 1-4 million nephrons per kidney [3] which are drained by a single collecting tubule. Multiple collecting tubules join in the medulla to form larger and larger ducts [2].
The functional unit of the kidney is the uriniferous tubule, a highly convoluted structure that modifies the fluid passing through it to form to form urine as its final output. This tubule consists of two parts, the nephron (Gr. nephros kidney) and the collecting tubule. There are approximately 1-4 million nephrons per kidney [3] which are drained by a single collecting tubule. Multiple collecting tubules join in the medulla to form larger and larger ducts [2].The Nephron
The nephron consists of a dilated portion, the renal corpuscle (located in the cortex); the proximal convoluted tubule; the thin and thick limbs of Henle's loop; the distal convoluted tubule; and the collecting tubules and ducts.
There are two types of nephrons, depending on the location of their renal corpuscles and the length of their Henle loop. (See Figure 2)
1. Cortical Nephrons
Shorter cortical nephrons have their renal corpuscles in the cortex and have short loops of Henle that do not go beyond the outer region of the pyramid. The thin segment in these nephrons is confined to a small part of the descending limb, and the distal thick segment begins at the hairpin turn [4].
2. Juxtamedullary Nephrons
These longer juxtamedullary nephrons have their renal corpuscles close to the base of a pyramid and have long loops of Henle with long thin segments. The thick descending limb does not extend beyond the outer stripe of the medulla. The thin limb begins here and extends deep into the inner region of the medullary pyramid. The thick ascending limb begins at a deeper level than that at which the thick descending limb ends. (In other words, the thin ascending limb is shorter than the thin descending limb.) [4]
The Renal Corpuscle
The renal corpuscle, an oval to round structure about 200 to 250 µm in diameter, is composed of a tuft of capillaries, the glomerulus, which is surrounded by a hollow capsule of tubular epithelium called Bowman's capsule.

The glomerulus is composed of tufts of fenestreated capillaries supplied by the afferent glomerular arteriole and drained by the efferent glomerular arteriole (Figure 4 above). The normal connective tissue cells are replaced by a specialized cell type know as mesangial cells. These provide the scaffold to support capillary loops. They also have contactile and phagocytic properties [5].
The capillaries constituting the glomerulus are similar to fenestrated type of capillaries. Their endothelial cells are highly attenuated, except for the region containing the nucleus, but the pores are usually not covered by a diaphragm [2].
Investing in the glomerulus is a basal lamina consisting of three layers. The lamina densa - the middle layer - is about 100 nm thick and consists of type IV collagen. The laminae rarae contain laminin, fibronectin and proteoglycans and are located on either side of the lamina densa. Lamina rara interna - between the endothelial cells of the capillary and the lamina densa and lamina rara externa - between the lamina densa and the visceral layer of Bowman's capsule.

The visceral layer of Bowman's capsule is composed of endothelial cells that are highly modified to perform a filtering function. These large cells, called podocytes, bear numerous long, tentacle-like cytoplasmic extensions, primary processes, which follow but usually do not come in close contact with the longitudinal axes of the glomerular capillaries. Each primary process gives rise to numerous secondary processes, called pedicels, that embrace the capillaries of the glomerulus by interdigitating with pedicels from neighoring processes of different podocytes forming the filtration slits [2,3]. These filtration slits, however, are not completely open; instead, they are covered by a thin slit diaphragm which acts as part of the filtration barrier [2].


The Proximal Convoluted Tubule
Bowman's space drains into the proximal convoluted tubule at the urinary pole. In this region the simple squamous epithelium of Bowman's capsule is continuous with the cuboidal, or low columnar epithelium of the proximal convoluted tubule (PCT). The PCT is the longest and widest part of the nephron, and constitutes the majority of tubular sections seen in the cortical labyrinth. It follows a tortuous course, then terminates by straightening out and passing into the nearest medullary ray to become the thick descending limb of the loop of Henle. The PCT is about 15 mm long, and the entire proximal thick segment can be up to 25 mm long [4]. The cuboidal cells of this epithelium contain numerous elongated mitochondria and thus have an acidophilic cytoplasm. The cell apex has abundant microvilli about 1 µm in length, which form a brush border. PCTs are surrounded by peritubular capillaries and associated with the apical cytoplasm of the cells are numerous canaliculi which increase the capacity of the PCT cells to absorb macromolecules. Pinocytotic vesicles are formed by the evagination of the apical membranes and contain macromolecule that have passed through the glomerular filter [3].
Sunday, March 2, 2008
Henle's Loop
 Henle's loop is a U-shaped structure consisting of a thick descending limb, a thin descending limb, a thin ascending limb and a thick ascending limb. The thin tubules are composed of squamous epithelial cells. The length of the thin segments varies with the location of the nephron. In cortical nephrons, the thin segment is only 1 to 2 mm long or may be completely absent. Juxtamedullary nephrons have thin segments, 9 to 10 mm in length, which form a hairpin-like loop that extends deep into the medulla as far as the renal papilla. It is possible to differentiate among four types of epithelial cells composing different regions of Henle's loop according to their fine structural features [2]. The locations and fine structural features of the four cell types are listed in Table 1.
Henle's loop is a U-shaped structure consisting of a thick descending limb, a thin descending limb, a thin ascending limb and a thick ascending limb. The thin tubules are composed of squamous epithelial cells. The length of the thin segments varies with the location of the nephron. In cortical nephrons, the thin segment is only 1 to 2 mm long or may be completely absent. Juxtamedullary nephrons have thin segments, 9 to 10 mm in length, which form a hairpin-like loop that extends deep into the medulla as far as the renal papilla. It is possible to differentiate among four types of epithelial cells composing different regions of Henle's loop according to their fine structural features [2]. The locations and fine structural features of the four cell types are listed in Table 1.The Distal Convoluted Tubule
The thick ascending limb of Henle's loop penetrates the cortex and is called the distal convoluted tubule (DCT). The DCTs differ from the PCTs in that they have no brush border, no apical canaliculi, and smaller cells.  DCT, like the ascending limb, is lined with simple cuboidal epithelium containing more or less round and apically located nuclei, having one or two dense nucleoli [2]. Cells of the DCTs have elaborate basal membrane invaginations and associated mitochondria indicative of their ion-transporting function [3]. Microvilli are present on the apical surface but they are much fewer in number and shorter than those of the proximal tubule [4]. As the ascending thick limb of the Henle loop passes near its own renal corpuscle, it lies between the afferent and efferent glomerular arterioles.
DCT, like the ascending limb, is lined with simple cuboidal epithelium containing more or less round and apically located nuclei, having one or two dense nucleoli [2]. Cells of the DCTs have elaborate basal membrane invaginations and associated mitochondria indicative of their ion-transporting function [3]. Microvilli are present on the apical surface but they are much fewer in number and shorter than those of the proximal tubule [4]. As the ascending thick limb of the Henle loop passes near its own renal corpuscle, it lies between the afferent and efferent glomerular arterioles. This region of the distal tubule is called the macula densa. The cells of the macula densa are columnar and their nuclei are closely packed together. Most of the cells have a Golgi complex in the basal region. These cells are sensitive to the ionic and water volume of the tubular fluid and lead to the release of the enzyme renin in the circulation [3].
This region of the distal tubule is called the macula densa. The cells of the macula densa are columnar and their nuclei are closely packed together. Most of the cells have a Golgi complex in the basal region. These cells are sensitive to the ionic and water volume of the tubular fluid and lead to the release of the enzyme renin in the circulation [3].
Juxtaglomerular Apparatus
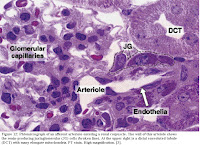 Together, the portion of the afferent arteriole containing the JG cells with the macula densa make up the juxtaglomerular apparatus [2,3]. Extraglomerular mesangial cells are also part of the apparatus and are coupled to one another and to JG cells by gap junctions. They are believed to be involved in transmitting information from the macula densa to JG cells [4]. The macula densa and the JG cells have a special geographical relationship in this area because the internal elastic membrane of the afferent arteriole is not present at this point, allowing intimate contact between cells of the macula densa and the JG cells [2,3].
Together, the portion of the afferent arteriole containing the JG cells with the macula densa make up the juxtaglomerular apparatus [2,3]. Extraglomerular mesangial cells are also part of the apparatus and are coupled to one another and to JG cells by gap junctions. They are believed to be involved in transmitting information from the macula densa to JG cells [4]. The macula densa and the JG cells have a special geographical relationship in this area because the internal elastic membrane of the afferent arteriole is not present at this point, allowing intimate contact between cells of the macula densa and the JG cells [2,3].
Collecting Tubules and Ducts
The collecting tubules are not part of the nephron as they have different embryological origins and only meet the nephron later in development to join it and make a continuous structure [4]. Urine passes from the DCTs to collecting tubules that join each other to form larger, straight collecting ducts which expand progressively as they near the tips of the medullary pyramids[3]. The permeability of collecting ducts is regulated by antidiuretic hormone (ADH). In its absence, such as in times of water excess they are impermeable to water. In its presence, when water in under-consumed, the opposite is true [4]. Collecting ducts are about 20 mm long and have three recognized regions.
1. Cortical collecting tubules are located in the medullary rays and are composed of two types of cubiodal cells: principal cells and intercalated cells. Principal cells have oval, centrally located nuclei and a few small mitochondria. The basal membrane of these cells contains numerous infoldings however do not interdigitate with neighboring cells. The lateral cell membranes are not plicated (folded). Intercalated cells have round, centrally located nuclei and an abundance of mitochondria. The apical cytoplasm contains numerous vesicles. Microplicae (cytoplasmic folds) and microvilli are also present on their apical surface. These cells do not have basal infoldings but do have basally located interdigitations with neighboring cells. Intercalated cells actively transport and secrete hydrogen ions against high concentration gradients and are thus involved in the acid-base balance of the body [2,4]. 2. Medullary collecting tubules are formed by the joining of several cortical collecting tubules and are thus larger than the above. In the outer region of the medulla, meduallry collecting tubules are similar to cortical collecting tubules in that they contain both principal and intercalated cells. Tubules of the inner medulla lack intercalated cells [2].
2. Medullary collecting tubules are formed by the joining of several cortical collecting tubules and are thus larger than the above. In the outer region of the medulla, meduallry collecting tubules are similar to cortical collecting tubules in that they contain both principal and intercalated cells. Tubules of the inner medulla lack intercalated cells [2].
3. Papillary collecting tubules (ducts of Bellini) are formed by the union of several medullary collecting tubules. These larger ducts, lined by tall columnar principal cells, open at the area cribrosa of the renal papilla to deliver the urine they carry into the minor calyx of the kidney [2].
 Calyces
CalycesA minor calyx is a funnel-shaped chamber which accepts urine from the renal papilla of a renal pyramid. The portion of the apex of the pyramid that projects into the minor calyx is covered by transitional epithelium. This type of epithelium separated urine from the underlying interstitial connective tissue, thus acting as a barrier. A muscular coat composed entirely of smooth muscle lies below the lamina propria. This layer forces the urine into a major calyx, a large funnel-shaped chamber, which recieves urine from two to four minor calyces. Major calyces are similar in structure and function to minor calyces and push the urine into the ureter which in turn delivers the urine to the urinary bladder for excretion [2,4].
Saturday, March 1, 2008
Renal Circulation

General Functions of the Kidney
Excretion and Resorption
The kidneys receive approximately 1220 ml of blood each minute, from which 125 ml/min of glomerular filtrate is formed in the average male. Thus, 180 L of glomerular filtrate is formed each day, of which only 1.5 to 2.0 L is excreted as urine. Therefore, every day at least 178 L is resorbed by the kidneys, and only about 1% of he total glomerular filtrate is excreted [2].
The fluid component from the blood passes through the filtration barrier to become the ultrafiltrate. The endothelial cells and basement membrane of the glomerulus as well as the podocytes of Bowman's capsule make up the filtration barrier. Blood enters the glomerular capillaries from an afferent arteriole and leaves through an efferent arteriole. Vasoconstriction of this efferent arteriole creates a high hydrostatic pressure in the glomerular capillary, forcing water, ions, and small molecules through the filtration barrier into Bowman's capsule. Whether a substance is filtered depends on both its molecular size and charge [5].
Most resorption of materials from the ultrafiltrate occurs in the proximal tubule. Normally, the following amounts are absorbed in the proximal tubule: 100% of proteins, amino acids, and creatine; almost 100% of bicarbonate ions; 67% to 80% of sodium and chloride ions; and 67% to 80% of the water [2]. Tables 3 and 4 below summarize the major function of all components of the uriniferous tubule.

 The excretory passages of the kidney consist of the minor and major calyces and the pelvis of the kidney which pass waste products along to the ureter, the single urinary bladder and subsequently on to the single urethra.
The excretory passages of the kidney consist of the minor and major calyces and the pelvis of the kidney which pass waste products along to the ureter, the single urinary bladder and subsequently on to the single urethra.Endocrine Function
The kidney plays a role in the endocrine system by producing four hormones as described below [5].
1. Renin is produced by the juxtaglomerular apparatus which causes the formation of angiotensin II. Angiotensin II acts on the proximal tubules to promote sodium retention and also acts as a potent vasoconstrictor.
2. Vitamin D is a steroid hormone metabolized by the kidney to the active form 1,25-dihydroxycholecalciferol, which promotes calcium and phosphate absorption from the gut.
3. Erythropoietin is a protein produced in the kidney which promotes red blood cell formation in bone marrow.
4. Prostaglandins are also produced in the kidney and have various effects, especially on renal vessel tone.
Because the kidneys are poised to sense plasma concentrations of compounds such as sodium, potassium, hydrogen ion, oxygen, and glucose, they are also important regulators of homeostasis including regulation of blood pressure, glucose metabolism, and erythropoeisis (the process by which red blood cells are produced).
